Lakhmir Singh Chemistry Class 10 Solutions For Chapter 1 Chemical Reactions And Equations - Free PDF
SOLVED: Use the following information to complete the table. Balanced equation: 2 Al(OH)3 + 3 H2SO4 → Al2(SO4)3 + 6 H2O Al(OH)3 H2SO4 Al2(SO4)3 H2O 88.003 g/mol 98.078 g/mol 342.15 g/mol 18.015
✓ Solved: When Al(OH)3 reacts with sulfuric acid, the following reaction occurs: 2Al(OH)3+3H2SO4→ Al2(SO4)3+6H2O...

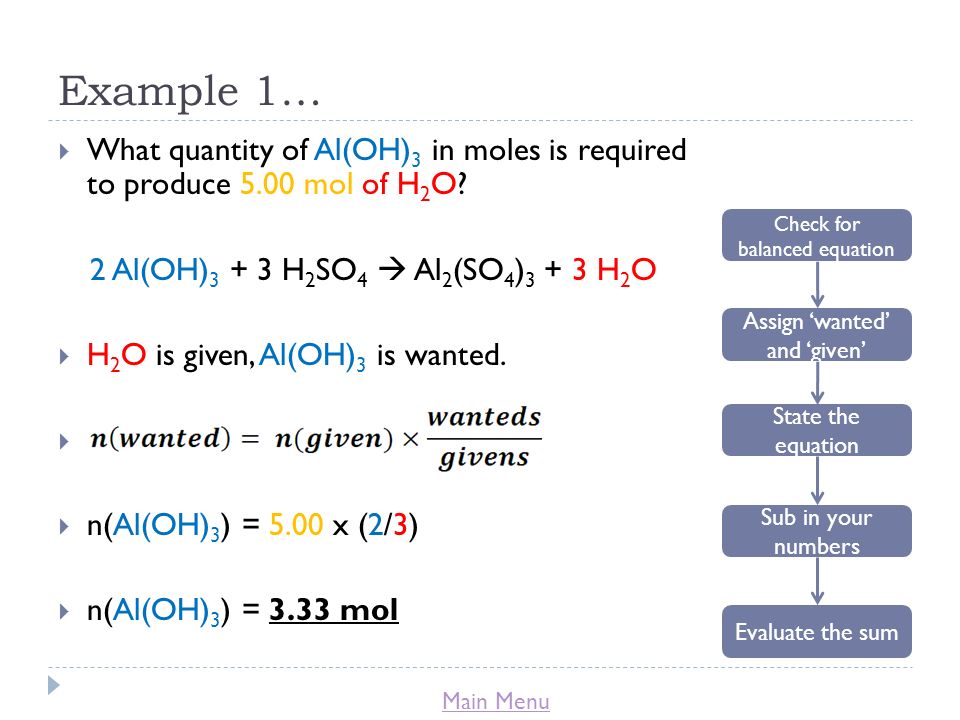
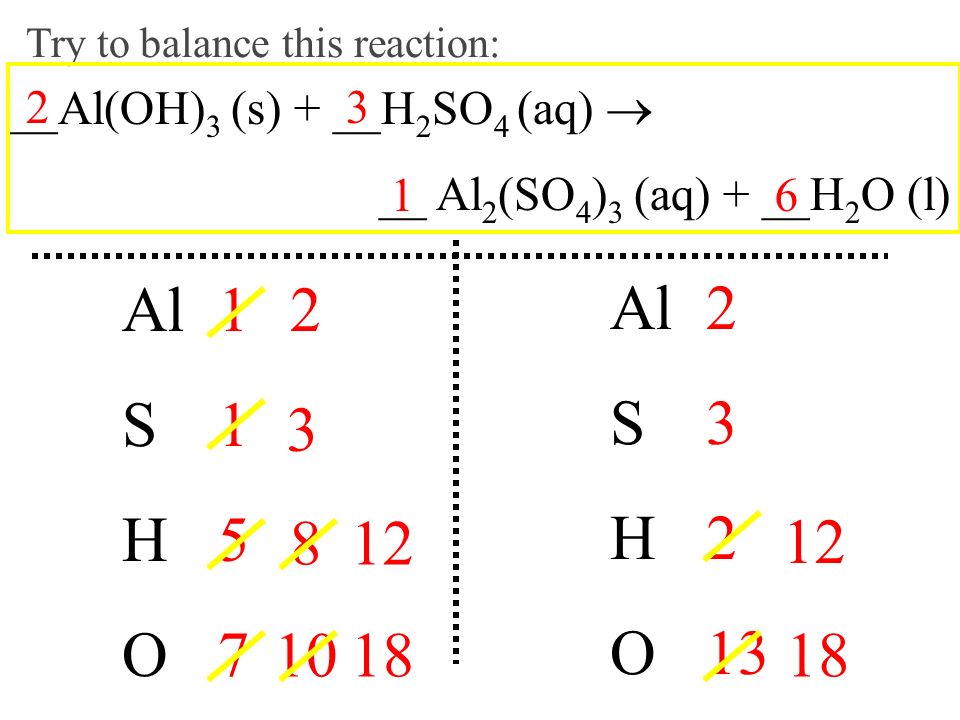
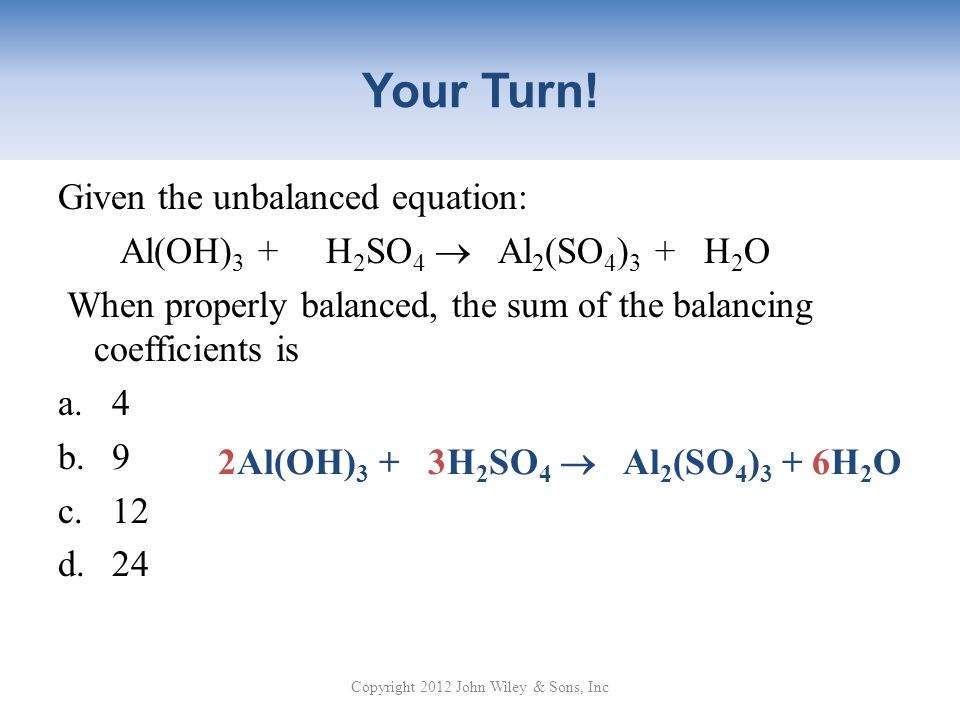
I. Balanced Equations Show Proportions. A. Relative Amounts in Equations Can be Expressed in Moles Stoichiometry -The branch of chemistry that deals with. - ppt download

Al(OH)3+H2SO4=Al2(SO4)3+H2O balance the equation @mydocumentary838. al(oh)3+ h2so4=al2(so4)3+h2o - YouTube
How to balance this redox reaction using the oxidation number method? Al(s) + H2SO4(aq) → Al2(SO4) 3(aq) + H2(g) - Quora

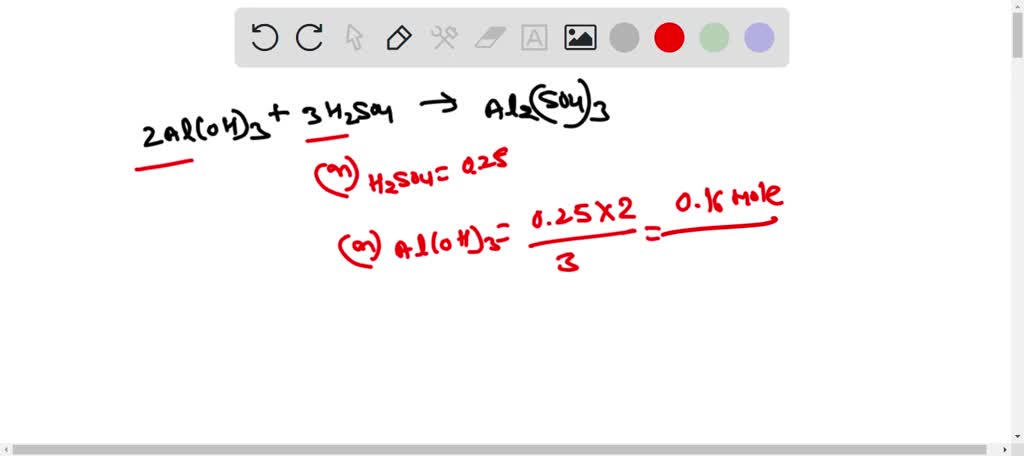
Alumminum hydroxide reacts with sulfuric acid as follows: 2Al(OH)3+H2SO4--> Al2(SO4)+6H2O. Which reagent is the limiting reactant when 0.500 mol Al(OH)3 and 0.500 mol H2SO4 are allowed to react? How ma | Homework.Study.com

H2SO4+Al(OH)3=Al2(SO4)3 +H₂O Balance| Aluminum hydroxide reacts with Sulfuric acid balanced Equation - YouTube